
Information for the Neuromuscular Community
Why is MOVR Important?
The Muscular Dystrophy Association (MDA) wants to collect information about individuals with neuromuscular disease to better understand the disease progression and ultimately improve the medical care, quality of life, and survival of those living with neuromuscular disease. To collect this information, MDA has created the Neuromuscular Observational Research Data Hub (referred to as the “MOVR Data Hub”). The MOVR Data Hub is a database, and serves as a platform for collecting and storing information.
The main reason the MOVR Data Hub is being set up is to monitor important trends in the population of individuals living with neuromuscular conditions, to improve understanding of the causes of disease, and to study the genetics (DNA) associated with neuromuscular conditions, disease progression, treatment, outcomes, and survival.
In addition, the MOVR Data Hub provides information for biomedical research and for the research and development of drugs, biologics, and medical devices. The MOVR Data Hub is also a source of information regarding potentially applicable clinical trials for interested individuals who may be eligible.
If you decide to take part in the MOVR Data Hub, information will be collected about you, your health, and other potentially relevant information by the MDA Care Center.
Each person who participates in the MOVR Data Hub will have his/her information collected for as long as the person is being seen at an MDA Care Center and information is still being collected, unless and until the person requests that the information no longer be provided to the MOVR Data Hub. If you are no longer being seen at an MDA Care Center, no additional information will be provided by the MDA Care Center to the MOVR Data Hub. If you request that information no longer be provided to the MOVR Data Hub, the MDA Care Center will stop providing your information to the MOVR Data Hub.
MDA’s goal is that at least 10,000 individuals living with neuromuscular diseases will participate in the MOVR Data Hub.
Information from the MOVR Data Hub might help health care providers, researchers, and companies developing and testing pharmaceuticals, biologics, and medical devices to better understand neuromuscular conditions and identify best practices for caring for and treating individuals with neuromuscular conditions.
Frequently Asked Questions
MDA’s mission is to empower the people we serve to live longer, more independent lives. MOVR aims to do just that through its strategic goals.
What are the goals of MOVR?

- Optimize Health Outcomes
- Support the development and refinement of standards of care
- Provide insights for quality improvement
- Inform clinical best practices
Accelerate Therapy Development
- Identify potential participants for clinical trials
- Identify clinical trial feasibility and design
Drive Understanding of Disease
- Characterize genotype-phenotype correlation
- Combine patient perspectives with genetic and clinical data sets
- Collect insights on pre-symptomatic infants identified via newborn screening
- Better characterize the patient experience
- Track natural history of disease
- Gather and assimilate data gathered outside a clinical trial (also known as real world data (RWD))
- Demonstrate how therapies are changing the course of disease
What is a registry?
A registry is a database of information that enables health care professionals to track or measure health-related or quality-of-life outcomes in individuals with a specific disease or condition.
What is a data hub?
A data hub is built on the classic registry model, adding data from patient reported outcomes, as well as from technological tools including smart phones, apps and wearables.
What indications are included in MOVR?
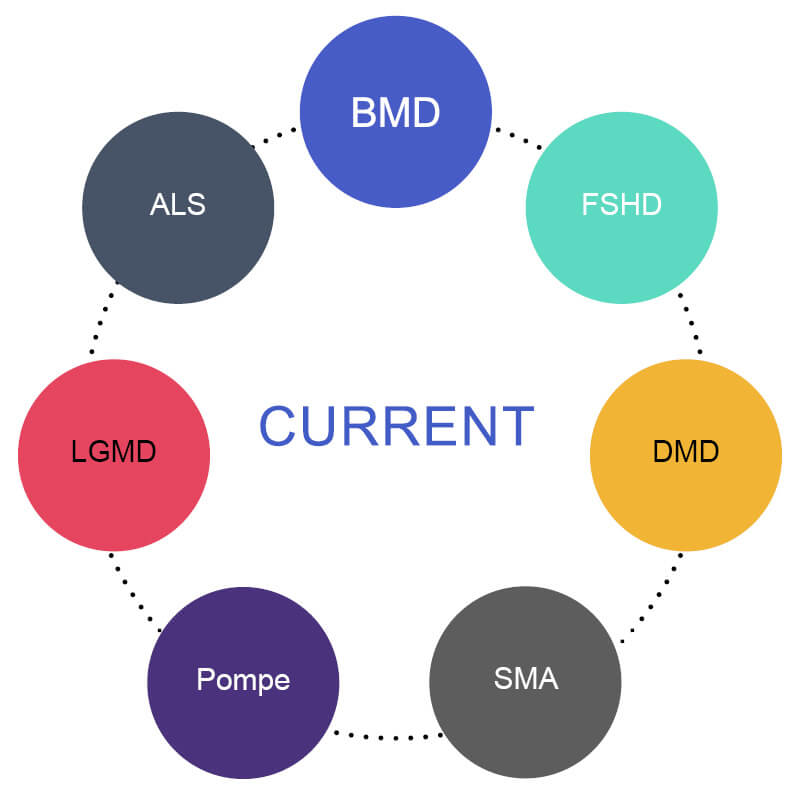
- Seven neuromuscular diseases:
- amyotrophic lateral sclerosis (ALS)
- Becker muscular dystrophy (BMD)
- Duchenne muscular dystrophy (DMD)
- Facioscapulohumeral muscular dystrophy (FSHD)
- Limb-girdle muscular dystrophies (LGMD)
- Pompe disease
- spinal muscular atrophy (SMA)
MDA is working to expand this list with the goal of capturing data from individuals living with any neuromuscular disease as time and funding allow.
Why does MOVR only include seven indications right now?
The seven neuromuscular diseases currently included in MOVR (ALS, BMD, DMD, FSHD, LGMD, Pompe disease, and SMA) were chosen because there are multiple experimental therapies in development for them, national working groups have identified and standardized the data elements that are important to collect in clinical trials, and formal “standards of care” for individuals living with these diseases have been defined. Importantly, this offers a unique opportunity to measure adherence to these care standards and their impact on individual health outcomes. MDA is committed to expanding the number of indications captured by MOVR.
What happens when a patient wants to participate in MOVR, but the hospital at which they receive care is not a MOVR site?
There is not an option to enroll patients outside of the designated MOVR sites. As the program grows, new sites will be added, and this will allow for additional patients to enroll. Patients and families, as well as interested sites are free to reach out to MDAMOVR@mdausa.org with questions.
What happens when patients want to be a part of MOVR and do not have a condition that data is currently collected for?
If a patient does not have one of the 7 diseases collected in MOVR, they are not able to participate at this time. As further information is gathered about neuromuscular diseases and as resources permit, MDA intends to add more disease case report forms to MOVR.
How is my data protected?
IQVIA is a life sciences organization who created this custom database for MDA. IQVIA uses industry IQVIA uses industry technology standards to implement administrative, physical, and technical safeguards to protect the availability, confidentiality, and integrity of Protected Health Information (PHI) and any other confidential data entered into the MOVR platform for a site. All such safeguards are in accordance with applicable federal, international, and state laws. If you’d like further detail around MOVR data security, please email MDAMOVR@mdausa.org.
Why did MDA create MOVR?
Diagnostic and therapeutic advances are transforming the landscape of neuromuscular medicine, with breakthroughs in neuromuscular disease research and care happening at a much faster pace than ever before. As more therapies become available, they will begin to shift in makeup from purely supportive treatments to those that modify disease at the molecular level.
In order to make upcoming therapies available to all individuals living with neuromuscular diseases, there are challenges that must be overcome.
MOVR aims to overcome these challenges by capturing clinical and genetic data from individuals living with a neuromuscular disease who receive care at an institution within the MDA Care Center Network and who agree to share their anonymized information. This large dataset will provide researchers with insights into how drugs and other treatments affect outcomes, how clinical trials could be better designed, and how neuromuscular diseases affect individuals the same or differently. It will ensure that clinicians can quickly identify patients who may benefit from new therapies or who may want to participate in a clinical trial.
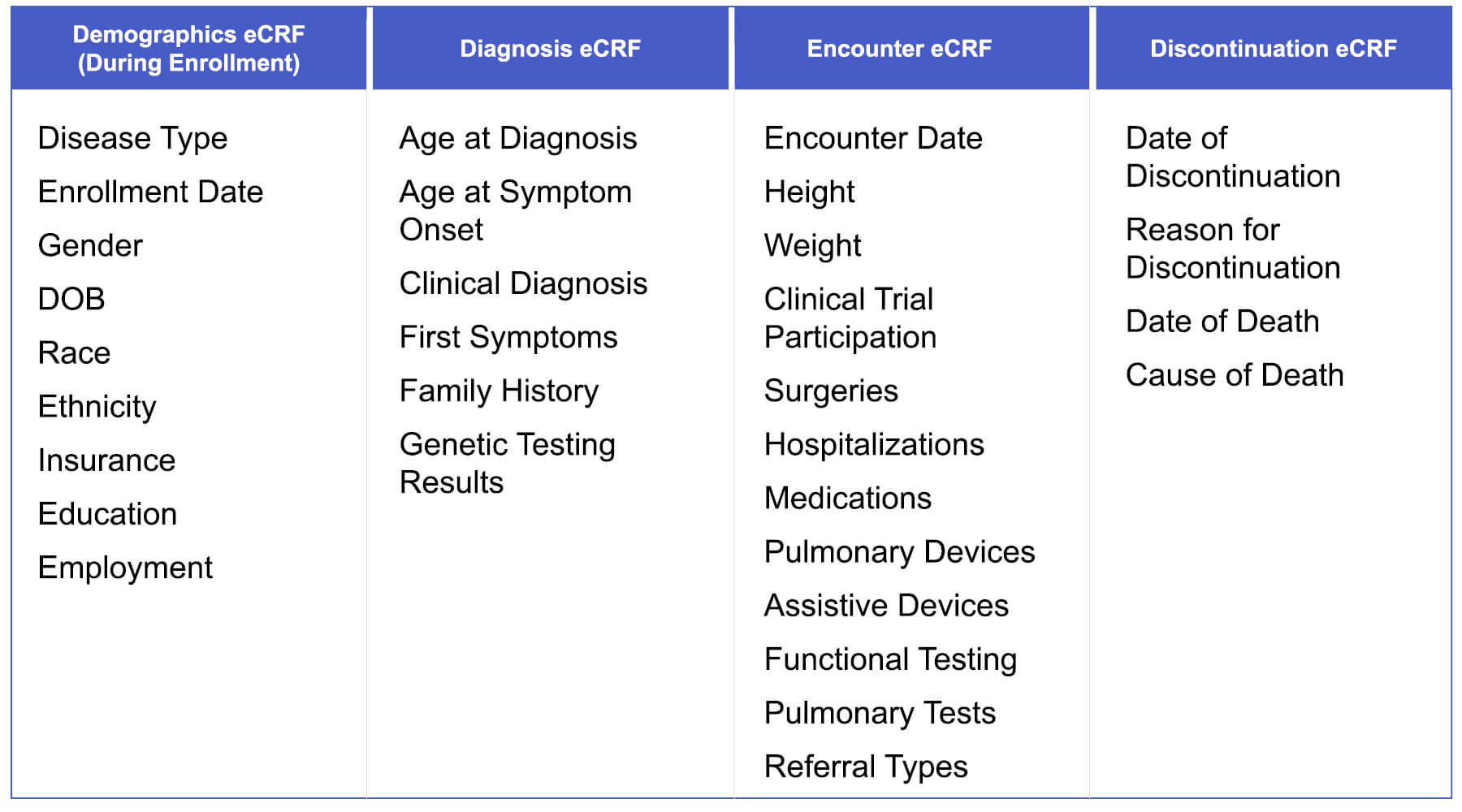
How are data entered into MOVR?

Thirty-one core data elements are captured across four electronic case report forms (eCRFs) – Demographics eCRF, Diagnosis eCRF, Encounter eCRF, and Discontinuation eCRF. Data are entered into the eCRFs from the electronic health record by clinical research staff at participating MDA Care Centers.
- MDA Medical Education
- Grants at a Glance
- Research Grants
- Creating a New Therapy
- What We've Achieved
- MDA Venture Philanthropy
- MDA Annual Conference
- MOVR Data Hub
- Newborn Screening for Neuromuscular Diseases
- Cost of Illness of Neuromuscular Diseases in the US
- Contact Our Research Team
- MDA Kickstart Program
- Telemedicine Resources
Find MDA
in your Community
-

Grants at a Glance
Read More -

Search for Clinical Trials
Learn More